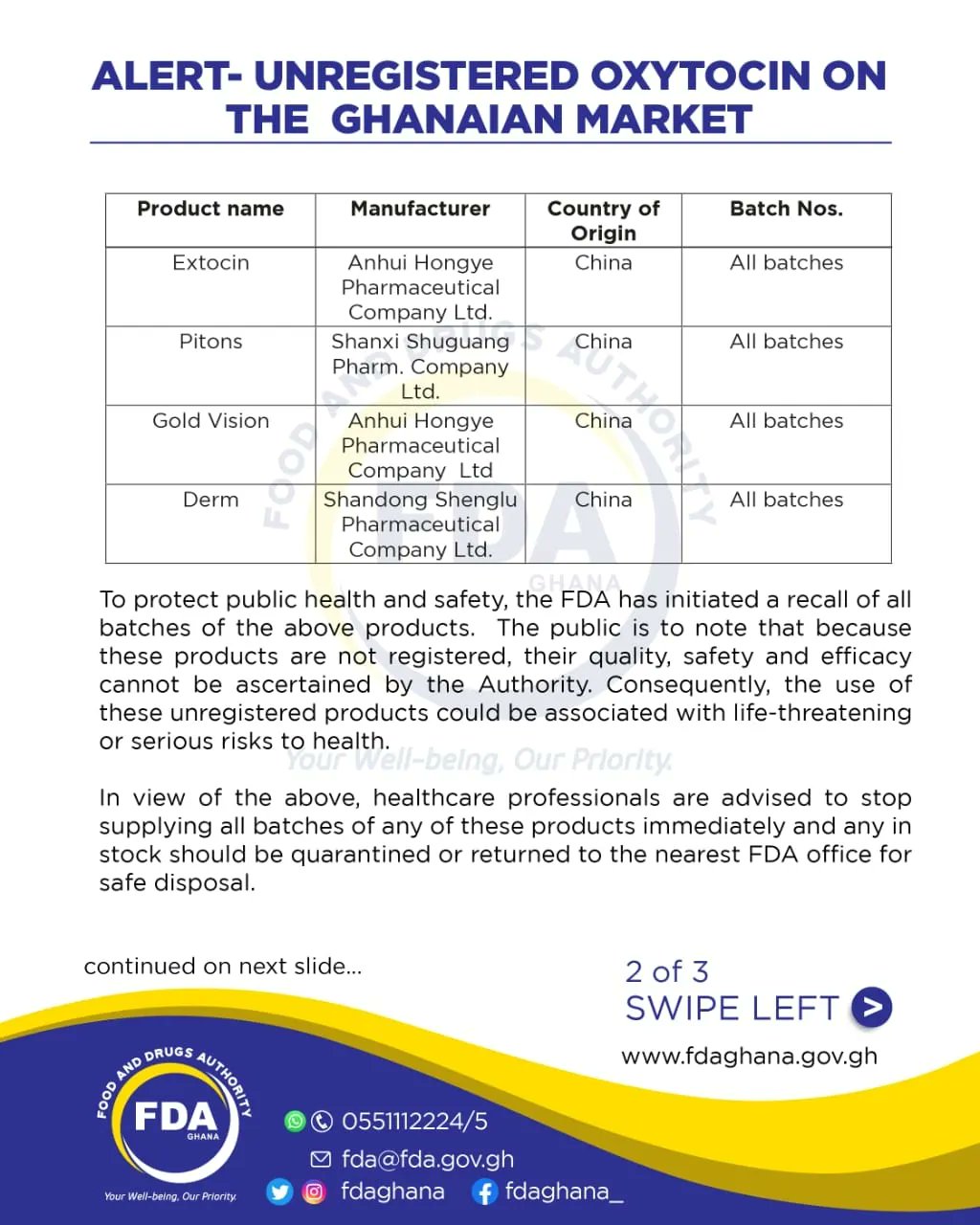
The Food and Drugs Authority (FDA) has cautioned the public that there is unregistered oxytocin circulating for sale on the Ghanaian market.
In a statement issued on Friday (21 April 2023), the FDA said “the pharmaceutical products are not registered with the FDA and therefore their quality, safety and efficacy cannot be ascertained”.
“The FDA is therefore informing all health facilities and medical stores of the above-mentioned products to stop using them immediately and return them to the nearest FDA offices throughout the country. Meanwhile, the FDA is liaising with importers to ensure that the market is rid of these unregistered pharmaceutical products,” the statement said.
“In this regard, the Authority is taking the necessary regulatory actions to prevent any such future occurrence,” the statement added.
However, the FDA has assured the public that it is taking all the necessary precautions to ensure that medical products on the Ghanaian market are safe, efficacious and of the right quality.