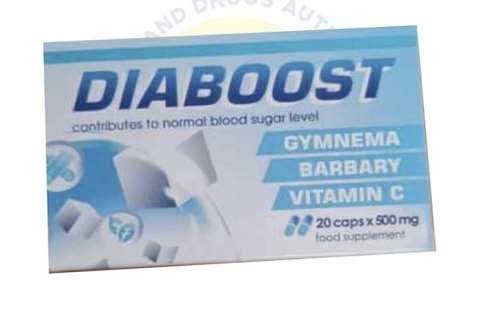
The Food and Drugs Authority (FDA) has detected the presence of an unregistered product, DIABOOST, marketed on social media by 36TY Courier Services, located at Ritz Junction, Madina.
The product is promoted as having the potency to cure diabetes and normalises blood sugar levels after first use.
“These claims are false. The product, labelled as a food supplement, not a medication and thus cannot be used in the treatment of diabetes,” it noted.
According to the FDA, DIABOOST is not registered by the Authority and for that reason, its efficacy, quality and most importantly, safety for diabetics cannot be guaranteed.
The Authority advised all diabetics to strictly adhere to the prescribed treatment by their doctors and discuss any alternative treatment with them to avoid any complications.
FDA hinted that DIABOOST is manufactured in Bulgaria for SPB, Realty Ltd, Drugg Han Asparuh Str., Burgas, Bulgaria and distributed in Ghana by 36TY Courier Service, headed by Ms Mary Alimena, who is currently assisting the Police with the investigation.
“The FDA is by this notice asking all persons who have the product to stop using them immediately and return them to the nearest FDA office or the supplier and encouraged the public to check the registration status of all regulated products from the FDA website, www.fdaghana.gov.gh,” it added.